Describe the Microscopic Perspective of Sodium Chloride.
See the answer Show transcribed image text Expert Answer. Sodium chloride is a readily soluble compound in water and other polar solvents and is a stable solid.
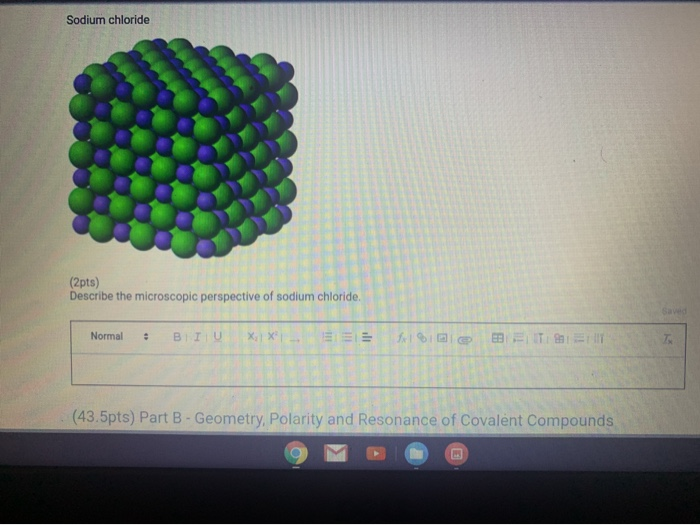
Sodium Chloride 2pts Describe The Microscopic Chegg Com
With molar masses of 2299 and 3545 gmol respectively 100 g of NaCl contain 3934 g Na and 6066 g Cl.
. The salient features of its structure are. Sodium Chloride is an ionic compound with the chemical formula NaCl. Electrically charged atoms called ions The sodium atoms are missing their outer electron.
Table salt is sodium chloride mixed with small amount of potassium iodide KI sodium iodide NaI or sodium iodate NaIO₃. The chlorine has one extra electron and its outer electron shell is complete so like sodium it too. It decomposes only at high temperatures to produce toxic fumes of disodium oxide Na2O and hydrochloric acid HCl.
Normal 2 BIU XX oil oil oil oil water water water water water Blank no solute With iodine dark purple solid With copper. Examine the chemical samples available at the dissecting microscope. Atomic level - sodium chloride NaCl has crystal cubic structure lattice-type arrangement with ionic bonds.
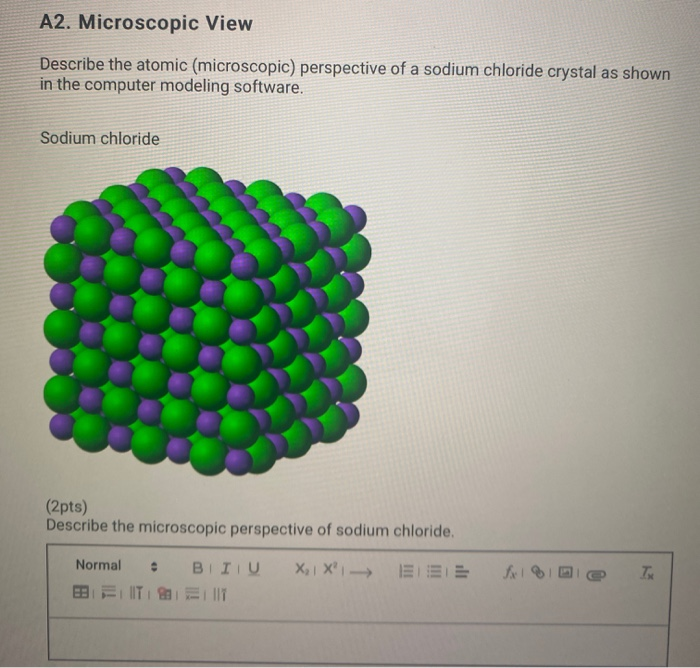
The Occurrence of Sodium Chloride. The three dimensional structure of NaCl shown below can be describe as cubic with chloride ions occupying the octahedral site and sodium ions located at the corners and centers of each face of the cube. It is a crystalline solid white.
Sodium chloride is obtained by mining the. Sodium chloride powder of 995 purity analytical grade by Merck was used as starting material. Uses of Sodium Chloride Of.
It was chosen because it focuses on structural features such as showing the cubical shape of a tiny crystal of sodium chloride displaying the charges of ions and the attraction of water to the ions and ending with a still image of the hydrated ions. In addition this CD-ROM accompanied the students textbook. With the chemical formula of NaCl salt crystals form lattices that repeat the specific arrangement of the two elements.
To have this salt crystallize so as to observe its crystals under the polarizing microscope the slide with the. Salt is a major ingredient of the dissolved materials in seawater. This indicates that it is a solid.
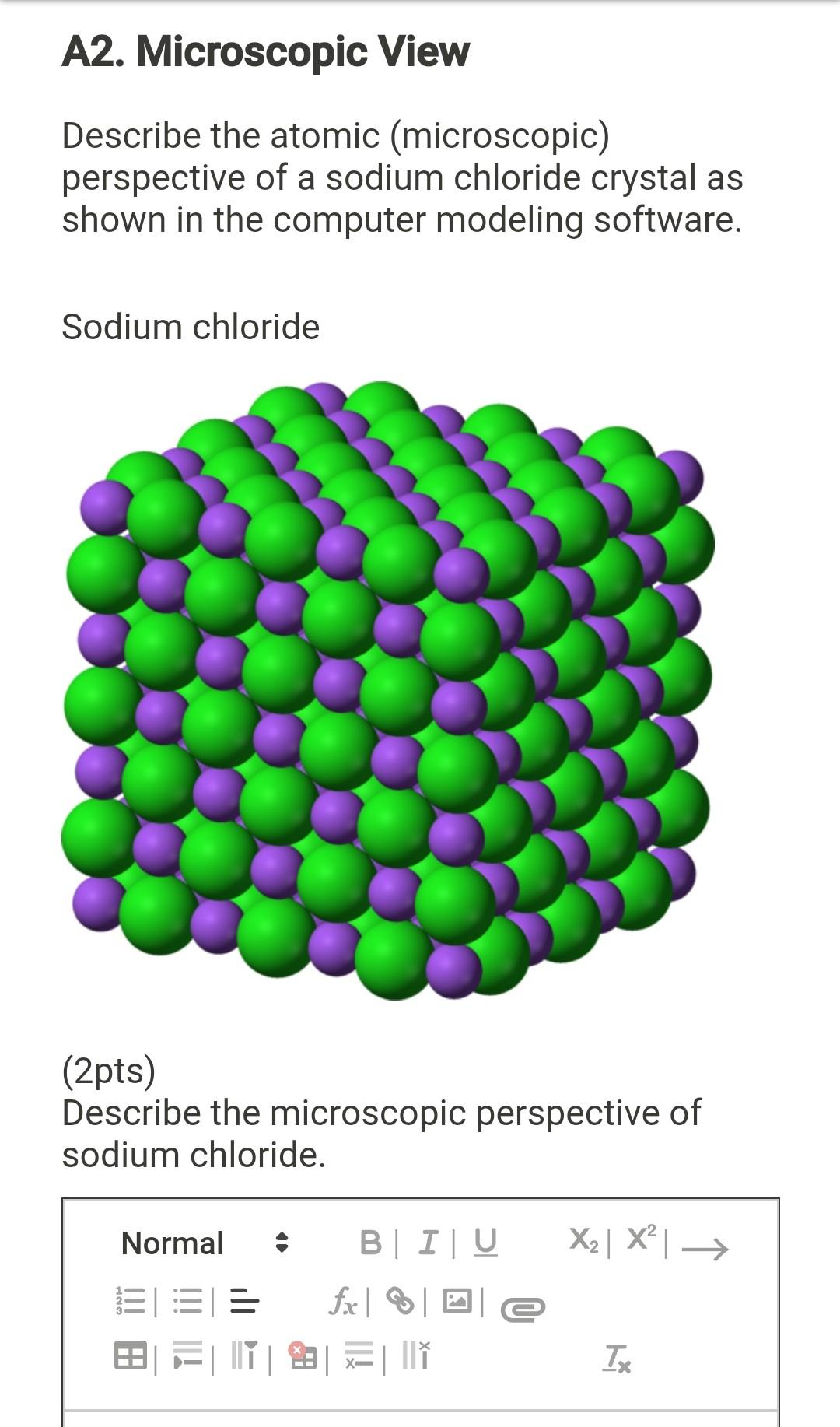
Microscopic View Describe the atomic microscopic perspective of a sodium chloride crystal as shown in the computer modeling software. Microscopic View Describe the atomic microscopic perspective of a Question. Because there are only one sodium ions for every.
A A still image from the PH animation in which the sodium and chloride ions are surrounded by water molecules. Chloride ions are ccp type of arrangement ie. In its aqueous form it is called a saline solution.
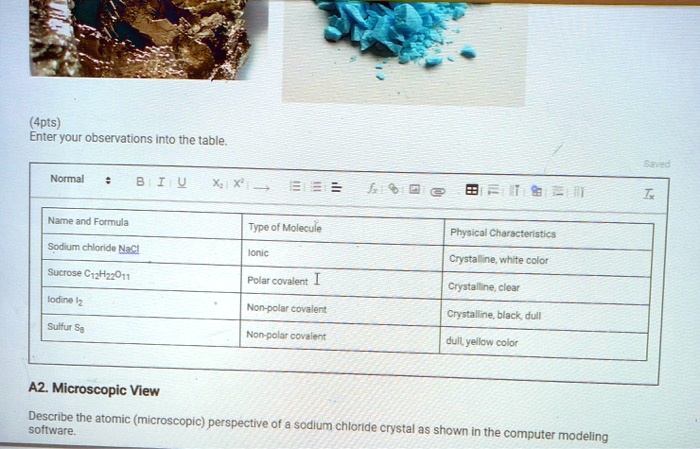
Positively charged ions attract negatively charged ions so finally the NaCl crystal consists of Na ions surrounded by six Cl-. Fx18 Name and Formula Type of Molecule Physical Characteristics Sodium chloride Naci Ionic Sucrose C12H22011 Polar covalent lodine 12 Non-polar covalent Sulfur Se Non-polar covalent A2. Sodium is cation with charge 1 and chlorine is an anion with charge 1-.
Consequently sodium chloride is the source of numerous other sodium compounds. The molecular weight of NaCl is 5844gmol. Fingerprint Salt Salt Aerosol Salt from Urine Sea Salt.
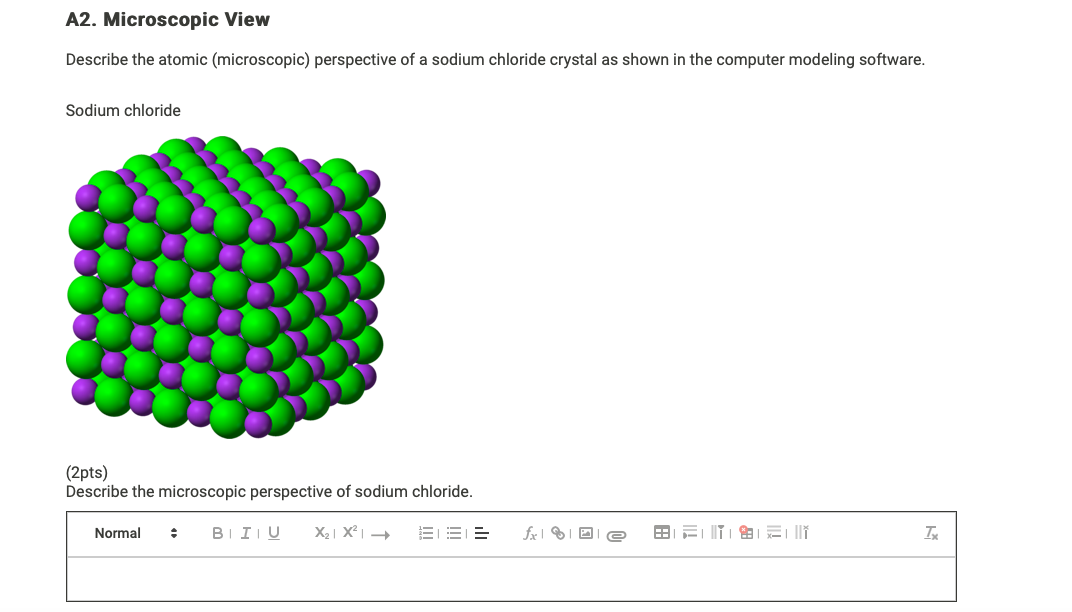
Sodium chloride 2pts Describe the microscopic perspective of sodium chloride. Table sugar sucrose iodine crystals and powdered sulfur. Microscopic View Describe the atomic microscopic perspective of a sodium chloride crystal as shown in the computer modeling software Sodium chloride 2pts Describe the microscopic perspective of sodium chloride.
The chemical formula of sodium chloride is NaCl. Sodium chloride or table salt is an ionic compound consist of 1 sodium ion and 1 chloride ion. The salt lattice model below demonstrates the ionic bonds and structure of the molecule.
Mostly all the chemical compounds which consist of chlorine or sodium is usually derived from salts. A large portion of the sodium chloride utilized is consumed in the production of sodium hydroxide Eqn 1123The production of sodium metal involves the electrolysis of. About 1 to 5 of seawater is made of NaCl.
Pure salt can be obtained from mineral halite. Lodine crystals Sulfur 4pts Enter your observations into the table. Microscopic View Describe the atomic microscopic perspective of a sodium chloride crystal as shown in the computer modeling software.
Sodium chloride 2pts Describe the microscopic perspective of sodium chloride. As the lattice is infinite I. The mean grain size was 50 µm.
As an Na atom approaches a Cl atom the outer electron of the Na atom is transferred to the Cl atom. Because NaCl is an ionic compound we dont talk about molecular geometry or. Sodium chloride is found in salt beds salt brines and sea water throughout the world and it is also mined in some locations.
The sodium chloride crystal is uniformly packed tightly together. And at the macroscopic and microscopic levels it will appear like this. How sodium chloride dissolves This surrounding of sodium and chloride ions by water molecules is called hydration.
It is also found as rock salt. Although the mechanism has not been conclusively determined some studies indicate that the drugs abortifacient activity may be mediated by prostaglandins released from decidual cells damaged by hypertonic solutions of sodium chloride. Sodium chloride also known as salt or halite is an ionic compound with the chemical formula NaCl representing a 11 ratio of sodium and chloride ions.
In this video well look at the crystalline structure of NaCl Sodium chloride. Sodium Chloride Under the Microscope Sodium Chloride Sodium Chloride is one of the most common chemical compounds encountered in environmental investigations. Intra-amniotic instillation of 20 sodium chloride injection induces abortion and fetal death.
The millimeter sized samples diameter 24 and 16 mm length for 6 mm anvil. The powder was pressed to a sample cylinder of 10 mm diameter and a length of 20 mm using a load of 6 tons resulting in an effective pressure of 02503 GPa. Therefore when we write Na aq or Cl aq the symbol aq aqueous usually means that each ion is attracted to and surrounded by several water molecules.
Sodium Chloride Salt is a clear-white mineral in the form of cubic crystals composed of two elements. BI ITU X 1 X fx 10 il e XII TX This problem has been solved. Because of this the remaining electrons behave as a filled electronic shell so they cannot easily react and form chemical bonds with other atoms except by electrical attraction.
The result is an Na and a Cl- ion. It may come from ocean spray perspiration mammalian urination fingerprints cooking mineral deposits and other sources. The figure is from Central Science.
Each ion is surrounded by opposite charged ions Table B1 Geometry and Polarity of Group T Compounds. Normal ill BIU X X BITIT T. Sodium Chloride is also known as salt.
Modeling Geometry and Polarity 7 Procedure Part A Macroscopic and Microscopic Views of Compounds 1. Describe the atomic microscopic perspective of a sodium chloride crystal as shown in the computer modeling software. Calcium chloride only crystallizes at relatively low relative humidity levels pure salts at a RH.
It is distributed abundantly in nature. It occurs in oceans and sea waters. Chemistry questions and answers.
Because the relative humidity on objects and in the laboratory usually lies above this value calcium chloride will only rarely crystallize on an object. They are sodium chloride an ionic salt and three covalently bonded substances.
Solved A1 Macroscopic View Examine The Images For Sodium Chegg Com

Solved Pts Enter Your Observations Into The Table Normal I F 3 K 0 5 8 Mi Name And Formula Tpe 0f Molecule Phyaical Characteristics Sodium Chloride
Belum ada Komentar untuk "Describe the Microscopic Perspective of Sodium Chloride."
Posting Komentar